Legionella pneumophila is a disease-causing microorganism. It is ubiquitous in water systems and can infect people. It is particularly dangerous for hospital patients with a weak immune system.
L. pneumophila is well known for causing Legionellosis (Legionnaire’s disease and Pontiac fever). The bacteria thrives in temperatures between 20 and 45°C and multiples rapidly in untreated or ineffectively treated water systems.
Legionella makes its way into a human system when it is caught by inhaling particles of aerosol emitted by showers and taps. These microorganisms have been responsible for many deaths. Therefore controlling legionella in water systems is vital, especially in hospitals and care homes.
In the UK Legionnaires’ disease must be reported to the Health Protection Agency (HPA). The total confirmed cases of Legionnaires’ disease in England and Wales reported to the HPA from 1980 to 2009 was 6750, of which 837 were fatal. Cases of deaths and resulting legal cases have been reported in the press from time to time. L. pneumophila was responsible for four Legionnaire’s disease deaths in Scotland during an outbreak in 2012, when 92 cases were registered (BBC News website http://www.bbc.co.uk/news/uk-scotland-edinburgh-east-fife-32216664).
Controlling Legionella In Water Systems
The most common control measures for Legionella are intermittent heat and flush, temperature control, chlorine dioxide and copper-silver ionisation. But, which method is the safest and most effective?
Heat and flush (thermal disinfection)
This is a ‘one-off’ approach and can be temporarily effective. However, it is expensive, energy inefficient and labour-intensive and, if not managed correctly, can present a major risk of scalding to both staff and patients. Research has shown that bacterial levels can soon return to pre-disinfection levels a few months after treatment resulting in further disinfection being necessary, which means further risk of scalding too.
Temperature control
The traditionally applied method for the control of Legionella in water distribution systems. This control method entailed obtaining 50ºC, and above, after running any hot water tap for 1 minute, and 20ºC, and below, after running any cold water tap for 2 minutes (HTM04-01, 2006). However, only 13% of the results of tests carried out by the UK Building Services Research and Information Association (BSRIA), in 1996, using temperature control were free of Legionella. In summary, it is not effective!
Intermittent shock injection of chlorine
Intermittent shock injection of chlorine into the water system, to achieve 20 to 50 mg/L of chlorine throughout the system, can be effective in the short term, however, bacteria re-colonisation often occurs after the disinfectant levels decrease (Lin et al. 1998). Protozoan cysts, such as amoeba, that harbour Legionella survive free chlorine levels of 50mg/L (Kilvington and Price, 1990). Chlorine also reacts with organic materials and accelerates the production of trihalomethanes (THMs), which are the only regulated disinfection by-product in the UK, and it is required by law that the sum of four THMs does not exceed 100 μg/L (Bougeard et al., 2010).
Chlorine Dioxide
The UK Drinking Water Inspectorate prescribe that you should not exceed 0.5mg/l of chlorine dioxide. However, maintaining between 0.3 and 0.5mg/l chlorine dioxide at outlets, which some studies with model systems demonstrated could control Legionella, is difficult as it decomposes to chlorite and chlorate and decays over distance and at elevated temperatures (Sidari et al., 2004). In comparison to chlorine dioxide, Chlorite and chlorate are not only toxic but also less active against Legionella and inactive against protozoa and biofilm that harbour Legionella.
There are health hazards to humans and environmental concerns associated with chlorine dioxide and to this effect, the UK Health Protection Agency has produced a guide on how to deal with chlorine dioxide incidents (https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/338711/Chlorine_Dioxide_Incident_management.pdf).
Some of the key points of the document are, chlorine dioxide:
• Reacts violently with organics, phosphorus, potassium hydroxide and sulphur, causing fire and explosion hazard.
• Emits toxic fumes of chlorine when heated to decomposition.
• Highly irritating via inhalation or ocular exposure.
• CHIP: Very toxic, corrosive and oxidising
• Inhalation causes irritation of eyes and nose with sore throat, cough, chest tightness, headache, ataxia and confusion. Dyspnoea and stridor due to laryngeal oedema may follow. Pulmonary oedema with increasing breathlessness, wheeze, hypoxia and cyanosis may take up to 36h to develop.
• Dangerous to the environment and you must inform the Environment Agency of substantial incidents.
Furthermore, chlorine and chlorine dioxide, being oxidising chemicals, create problems in older distribution systems by causing a pitting type of corrosion.
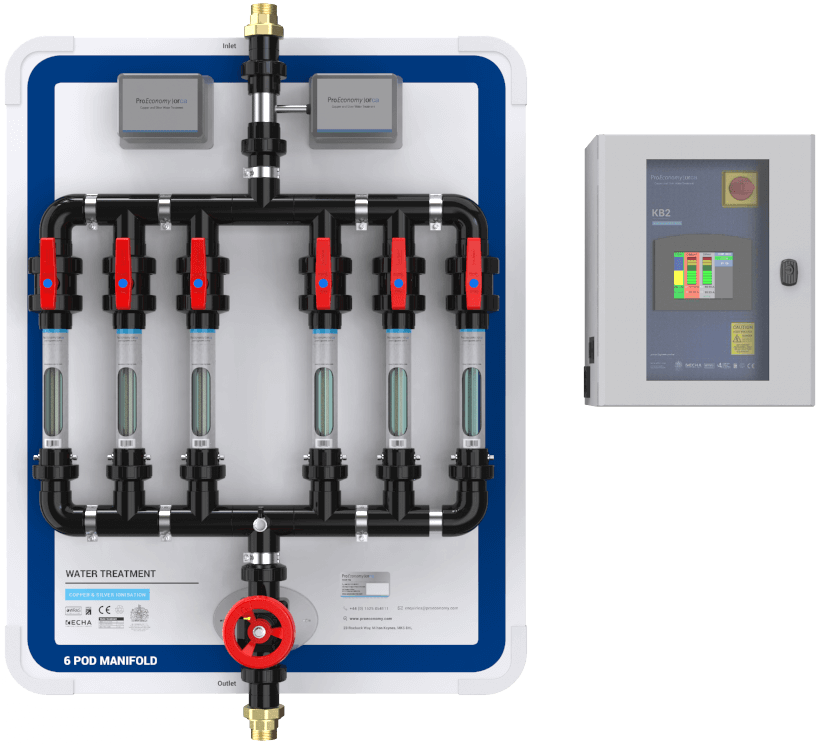
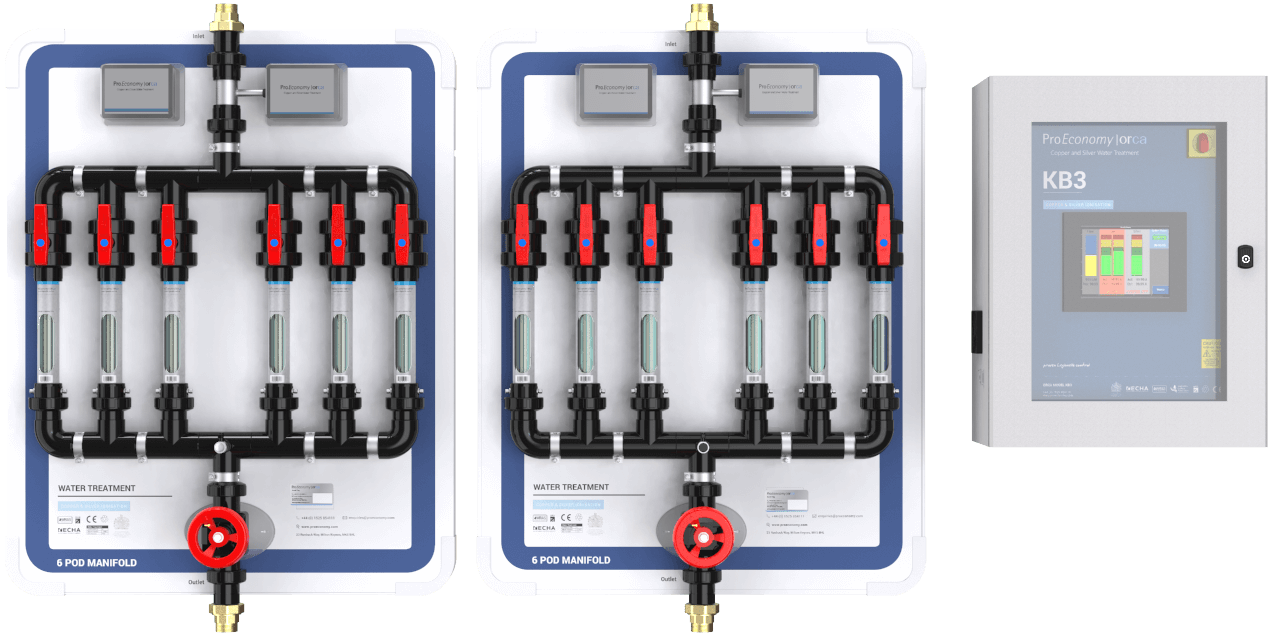
Copper and silver ionisation
CSI involves the continuous release of copper and silver ions in water. We generate the ions by passing a low electrical current between two pure copper and two pure silver electrodes or between two copper and silver alloys (normally 70:30 ratio).
Copper and silver ions are positively charged ions (cations). Therefore, they seek opposite polarity and find this in Legionella bacteria as well as in biofilm. The copper and silver ions attach, through electrostatic bonds, to negatively charged sites on bacterial cell walls. Consequently, this distorts and weakens the cell wall allowing penetration of the silver ions. Then the silver ions attack the cell by binding at specific sites to DNA, RNA, cellular protein and respiratory enzymes. Consequently, this denies all life support systems to the cell, causing death. The copper and the silver ions need to work together, since without the copper ions the silver ions cannot penetrate the cell wall of the target organisms. (See also previous blog on the Orca system).
The UK Regulations do not specify a maximum concentration value for silver, but the US Environmental Protection Agency (EPA) has set maximum containment levels for drinking water of 1.3mg/l for copper and 0.1mg/l for silver (Lin et al., 2011). The levels of copper and silver needed to eradicate Legionella in water are much lower than that (<0.2 mg/L copper and 0.02 mg/L silver). A long-term study (5 – 11 years) on the efficacy of copper and silver ionisation to control Legionella in 16 hospital water systems, to reduce incidence of hospital-acquired Legionnaires’ disease concluded that copper and silver ionisation was the only disinfection modality to have fulfilled all criteria recommended to be applied to disinfection approaches (Stout and Yu, 2003).
Advantages Of Copper and Silver Ionisation
Various hospital studies have shown copper and silver ionisation to be effective against Legionella species and Pseudomonas bacteria. The main advantages of copper and silver ionisation are:
- Precision, as the ions generation is controlled by the water flow, so it can be adjusted to the required level
- It has a residual effect
- Penetrates biofilms
- Works at a range of water temperatures
- You only need tiny amounts (20 to 40 ppb silver and 200 to 400 ppb copper) to achieve Legionella control
- More stable than ClO2, for example, which gases off.
- Most importantly, copper and silver ionisation is much safer than chlorine-based chemicals that can explode in situ, during transport and during disposal of drums with small amounts of the chemical in them, as shown in the last article below.
In conclusion, the only method of controlling legionella in water systems which matches safety and effectiveness, seems to be copper-silver ionisation. ProEconomy offer advanced water treatment and monitoring with our copper silver ionisation system, The Orca.
References
Bougeard CMM, Goslan EH, Jefferson B, Parsons SA. Comparison of the disinfection by-product formation potential of treated waters exposed to chlorine and monochloramine. Water Research 2010;44(3):729-740.
Kilvington S, Price J. Survival of Legionella pneumophila within cysts of Acanthamoeba polyphaga. Journal of Applied Bacteriology 1990;68:519-525.
Sidari FP, Stout JE, van Briesen JM. Keeping Legionella out of water systems. J. Am. Water Works Assoc. 2004;96:111-119.
Stout JE, Yu VL. Experiences of the first 16 hospitals using copper silver ionisation for Legionella control: Implications for the valuation of other disinfection modalities. Infection Control and Hospital Epidemiology 2003;24(8):563-568.