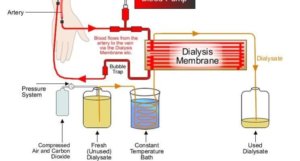
 Considering that approximately 400 litres of water is used weekly for producing dialysis fluid, it is important to know and monitor the chemical and microbiological purity of dialysis water (Pontoriero et al 2003). Hospitals’ dialysis units have to deliver high purity water, which is free from contaminants. Therefore, metals as well as chemical compounds should be removed from the water for dialysis. To ensure water is free from impurities, additional treatment of drinking water is done by means of filtration, including reverse osmosis.
Considering that approximately 400 litres of water is used weekly for producing dialysis fluid, it is important to know and monitor the chemical and microbiological purity of dialysis water (Pontoriero et al 2003). Hospitals’ dialysis units have to deliver high purity water, which is free from contaminants. Therefore, metals as well as chemical compounds should be removed from the water for dialysis. To ensure water is free from impurities, additional treatment of drinking water is done by means of filtration, including reverse osmosis.
What Will Contaminate Dialysis Water Filtration Systems?
Water used for the preparation of dialysis fluid should be ideally ultrapure water or at least meet the minimum standard of purity given in Table 1 (Mactier 2007). The table includes contaminants that should always be included in routine testing because they occur in relatively high levels and are not restricted in drinking water, those for which drinking water limit is more than five times the recommended limit for water for dialysis, and all contaminants for which the drinking water limit is 2 to 5 times the recommended limit for dialysis.
In water treated by reverse osmosis, these contaminants will only exceed the limits in table 1 if they occur at relatively high levels in the water supplied to the unit. These contaminants can be omitted from routine tests if data is available to show that the levels in the water supplied to the unit rarely exceed the limit in the table. These data should be obtained from the municipal water supplier or from tests on the raw water if it is obtained from a private source (Mactier 2007).
Table 1: Maximum recommended concentrations for chemical and microbial contaminants in water for dialysis
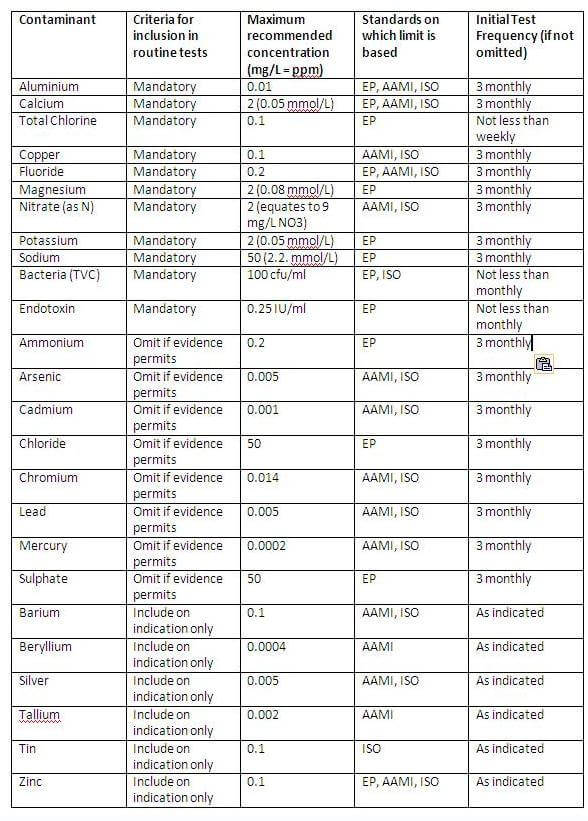
Note: Antimony (AAMI limit 0.006 mg/L) and selenium (AAMI and ISO limit 0.09 mg/L) have been excluded from this table as the limit for drinking water in the UK is lower than the limit for water for dialysis.
As shown in Table 1, contaminants of dialysis water filtration systems include the chemical elements from the periodic table that are widely used in products and processes and thus end up in water courses (e.g. aluminium, calcium, potassium, sodium, magnesium, chlorine, chloride, fluorine, fluoride, copper, cadmium, lead, mercury, silver, tin, zinc); chemical compounds (e.g. nitrate, ammonium, sulphate) as well as bacterial contaminants (e.g. bacteria, TVCs, endotoxins). Therefore, removal of these contaminants from water has to be carried. This achieves the maximum concentrations of chemical and microbial contaminants allowed in dialysis water. This is usually done by filtration.
Table 1 is from the Renal Association’s document “Clinical Practice Guidelines Module 2: Haemodialysis”. It shows the list of possible contaminants and their maximum recommended concentration in water for dialysis. If the filtration equipment fails then contamination of the dialysis water and the patient’s blood could occur.
Which Legionella control method is less likely to contaminate Dialysis Water filtration systems?
The dialysis filtration system can fail if using compounds like silver hydrogen peroxide as a biocide to control Legionella in the water system, as happened in Leicester General Hospital some years ago (Martin 2008). The filtration system was not able to cope with the high concentration of silver hydrogen peroxide in the water. Therefore, this resulted in contamination of the dialysis water.
This would not have happened if the hospital was using Copper and Silver Ionisation. With CSI, the dialysis water filtration system would have been able to cope without any problems. This is because the levels of ions of copper and silver released into the water by the copper and silver ionisation system to control pathogens like Legionella and Pseudomonas aeruginosa are extremely small (0.200-0.800 mg/L for copper and 0.020-0.080 mg/L for silver).
Sometimes people get confused with these two systems. Therefore, I will point out that there is a huge difference between copper and silver ionisation and silver hydrogen peroxide. In fact, the two modalities are completely different. The concentrations of silver salts (not ions) used in silver hydrogen peroxide are between 8 to 12 mg/L (more than 400 times the level used in Cu-Ag ionisation).
Copper and Silver Before and After Dialysis Water Filtration
ProEconomy Ltd, who are the only providers of the Orca pure copper and silver ionisation system for pathogens control in water has carried out analysis to measure levels of copper and silver before and after dialysis water filtration equipments and the results are shown in Table 2, where it can be seen that the copper concentration before the RO unit was 0.246 mg/L and after the unit was <0.003 mg/L, showing almost complete removal to well below the recommended levels (0.1 mg/L). The silver concentration before the unit was 0.059 mg/L and after it was 0.0004 mg/L, which again shows almost complete removal to well below the allowed levels (0.005 mg/L) as shown in Table 1.
Table 2: Copper and Silver Before and After Dialysis Water Filtration
| Sample Id | Copper (mg/L) | Silver (mg/L) |
| Maximum recommended concentration (Renal Association, AAMI-ISO) | 0.1 | 0.005 |
| Renal Dialysis Before osmosis | 0.246 | 0.0593 |
| Renal Dialysis After osmosis | <0.003 | 0.0004 |
References
Mactier, R. (2007) Clinical Practice Guidelines Module 2: Haemodialysis. UK Renal Association, 4th Edition, Final Version. https://renal.org/module_3a_-_haemodialysis_-_4th_edition/http://www.renal.org/docs/default-source/guidelines-resources/Module_3a_-_Haemodialysis_-_4th_Edition.pdf?sfvrsn=0
Martin, D. (2008) The ‘deadly threat’ to kidney dialysis patients from hospital water supply. Mail online http://www.dailymail.co.uk/health/article-1081353/The-deadly-threat-kidney-dialysis-patients-hospital-water-supply.html#ixzz4L07RkxS0
Pontoriero, G., Pozzoni P, Andrulli S, Locatelli F (2003) The quality of dialysis water. Nephrol Dial Transplant. Aug;18 Suppl 7vii:21-5; discussion vii56. http://www.ncbi.nlm.nih.gov/pubmed/12953025