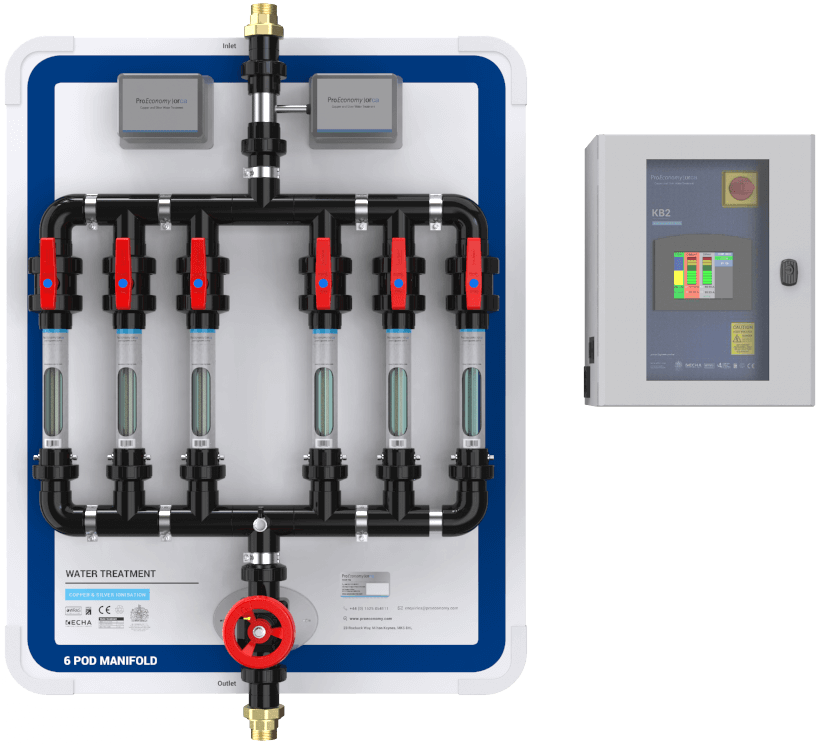
How The Orca Works
The most advanced copper and silver water treatment
Incoming water first flows through the turbine of a flow sensor. This sends a signal to the Orca control unit, which then passes a low DC current between two copper and two silver electrodes located in an electrode chamber (the ‘Pod').
The current causes the release of electrically charged copper and silver ions from the electrodes. The system is designed so that ion release is proportional to the flow rate, maintaining a constant concentration.
When the positively charged ions meet bacteria, it’s thought that copper reacts with negatively charged molecules on their surface, weakening the wall and allowing silver ions to enter. The silver (and copper) ions then react with biomolecules such as DNA and enzymes, killing the bacterium.
The copper and silver gradually cause the breakdown of any biofilm present in the system. If this didn’t happen Legionella and Pseudomonas would quickly return.
Download our Orca brochure
Simply fill in the form below to download our brochure.What makes the Orca better than most copper-silver ionisation systems?
Many things, but a key reason is the fact that we only use 99.99% pure electrodes rather than copper/silver alloy ones. Research has shown that alloy electrodes under-dose the silver, heavily reducing the system’s effectiveness. Pure electrodes don’t have this problem.
To quote a recent research paper, “Silver can only be dosed accurately with copper and silver ionisation systems using separate high purity silver electrodes.”**
It is because of this that our Orca copper silver ionisation system is effectively controlling Legionella at prestigious sites worldwide.
** Walveren, N., Pool, W. and Chapman, C., (2015). The dosing accuracy of copper and silver ionisation systems: separate high purity copper and silver electrodes versus copper/silver alloys. Journal of Water Process Engineering, Vol. 8, p.119-125.
Advantages of Copper Silver Ionisation
- Good distribution
UV, ultrasound and filtration only treat water at the point of contact. The Orca, however, distributes active copper and silver ions throughout the whole system and into the biofilm, which it breaks down.
ReferenceShih, H.-Y and Lin, Y.E., (2010), Efficacy of Copper-Silver Ionization in Controlling Biofilm and Plankton-Associated Waterborne Pathogens, Applied and Environmental Biology, Mar; 76(6): 2032-2035"In summary, copper-silver ionisation is efficacious for control of biofilms and plankton associated waterborne pathogens in a model plumbing system. Copper-silver ionization may be capable of controlling waterborne pathogens, in addition to Legionella, in the hospital water distribution system.*"
*Data collected by ProEconomy over the last twenty years show that this is indeed the case.
- Residual Efficacy
Copper and Silver ions remain active until they are consumed, so have long-lasting effect. Furthermore, unlike chlorine dioxide they aren't affected by temperature and don't decay or 'gas off' over distance.
ReferenceLin, Y.E., Stout, J.E., & Yu, V.L., (2011), Controlling Legionella in Hospital Drinking Water: An Evidence-Based Review of Disinfection Methods, Infect Control Hosp Epidmiol 32(2):166-173."When the ionization unit was deliberately inactivated, recolonization was delayed, and the water system remained free of Legionella for an additional 2-3 months."
Ionization has a prolonged efficacy that provides an added margin of safety, unlike hyperchlorination, with which Legionella can rapidly appear in the even of system malfunction. Unlike chlorine and chlorine dioxide, the biocidal activity of copper-silver ionization is not compromised by higher water temperature."
- Safe and Hazard free
The copper and silver levels found at outlets are safe to consume - there are no known harmful effects on humans or animals at these concentrations.
Copper and silver electrodes are safe to handle, store and transport - COSHH compliance is not an issue.
ReferenceWHO Guidelines for drinking water quality, 4th edition, page 415"Only a small percentage of silver is absorbed. Retention rates in humans and laboratory animals range between 0% and 10%.
The only obvious sign of silver overload is argyria, a condition in which skin and hair are heavily discoloured by silver in the tissues. An oral NOAEL for argyria in humans for a total lifetime intake of 10g of silver was estimated on the basis of human case reports and long-term experiments with laboratory animals.
The low levels of silver in drinking-water, generally below µg/l, are not relevant to human health with respect to argyria. In contrast, special situations exist where silver salts may be used to maintain the bacteriological quality of drinking-water. Higher levels of silver, up to 0.1 mg/l* (this concentration gives a total does over 70 years of half the human NOAEL of 10 g), could be tolerated in such cases without risk to health."
*The Orca system never exceeds 0.08 mg/l
- Non-corrosive
Copper and Silver ionisation is non-corrosive to pipework. ProEconomy has never encountered pipework corrosion of the orca system in more than twenty years of operating systems across over 200 sites.
- Scientifically proven to work
Study after study carried out by scientific institutions have shown its efficiency at killing bacteria. The Orca system has eliminated Legionella in every water system in which it has been installed, including those where other control methods such as chlorine dioxide had been tried and failed. What's more, the Orca system not only kills Legionella, it also gets rid of other disease-causing microbes such as Pseudomonas, E.coli and Salmonella.
ReferenceScottish Health Technical Memorandum 04-01 p.17-18"A laboratory study by Huang et al. (2008) of copper and silver ions in combination provided evidence to suggest that bactericidal efficiencies are greater than 99.99% against the most significant clinical waterborne microbes; P. aeruginosa, Acinetobacter baumannii, and S. maltophilia, including Legionella."
- Temperatures can be reduced
Recommended temperatures for killing Legionella are difficult and expensive to maintain in complex water systems.
Reference"With large centralised hot water systems, it is more difficult to maintain secondary distribution temperatures within recommended values; also, water flow rates in large secondary distribution systems can prove difficult to balance."
Scottish Health Technical Memorandum 04-01, 2011, Para. 2.11, p.13.
"The energy costs of maintaining a hospital hot water system above 60°C are substantial and may not reliably prevent persistence at parts of the system where there is infrequent use and a lower temperature."
The Orca system kills Legionella and Pseudomonas regardless of water temperature, so these can be reduced. The Health & Safety Executive state that once a system has been installed the temperature can be gradually brought down in commercial premises. This removes the need for expensive mixing valves that can act as a source of contamination.
- Effective at any Water Temperature
The copper and silver ions produced by the Orca system can control Legionella and Pseudomonas regardless of temperature. This makes the Orca system an excellent secondary mobility to supplement the temperature control regime and also makes it a great choice for sites that struggle to maintain consistent water temperatures across their site.
European Compliance
Status - fully compliant
All organisations adding biocides to drinking water must comply with the European Biocidal Products Regulations 528/2012 (EU BPR). Companies have to apply for approval of a biocidal substance by submitting a technical dossier of evidence to the ECHA (European Chemicals Agency).
From 1 September 2015, a biocidal product consisting of, containing, or generating a relevant substance, cannot be make available on the EU market if the substance supplier or product supplier is not included in the list for the product type to which the product belongs. This is known as the Article 95 List. ProEconomy is listed on Article 95 for both copper and silver.
ProEconomy is a founder member of the task forces of European companies set up to produce the dossiers for copper and silver. These dossiers have now been completed and are currently being evaluated by the designated member states.
Understand Article 95Health & Safety Executive Compliance
Status - fully compliant
ProEconomy is fully compliant with all HSE recommendations and guidance.
These publications are for dutyholders, which includes employers, those in control of premises and those with health and safety responsibilities for others, to help them comply with their legal duties. These include identifying and assessing sources of risk, preparing a scheme to prevent or control risk, implementing, managing and monitoring precautions, keeping records of precautions and appointing a manager responsible for others.
- Legionnaires' disease. The control of Legionella bacteria in water systems. Approved Code of Practice and Guidance- Download a copy
- Legionnaires' disease. Technical Guidance (HSG 274) - Part 1 - Part 2 - Part 3
- Control of Legionella bacteria in water systems. Audit Checklists- Download a copy
Operational Compliance
Health & Safety
We hold the ConstructionLine SafeContractor certificate
Quality Management
ProEconomy meets the requirements of ISO9001
Department of Health Compliance
Status - fully compliant
ProEconomy is fully compliant with all DoH recommendations and guidance.
The Health Technical Memorandum gives advice and guidance on the legal requirements, design applications, maintenance and operation of hot and cold water supply, storage and distribution systems in all types of healthcare premises.
It also provides advice and guidance on the control and management of the risk posed by Legionella, Pseudomonas Aeruginosa and other water borne pathogens within a healthcare setting.
Safe Water in healthcare premises
- Part A - design, installation, commissioning - Download a copy
- Part B - operational management - Download a copy
- Part C - additional measures to minimize Pseudomonas risk in augmented care units - Download a copy
- Part D - performance specification, thermostatic mixing valves - Download a copy
FAQs
- Isn't using copper as a biocide banned by the EU?
The answer is NO. Copper was taken off the market in2012 as the copper industry hadn't formed a task force as required by the Biocidal Products Directive. This has now been done, with ProEconomy a founding member. Therefore, copper is completely legal and compliant for use in Europe.
ReferenceImportant information for users and suppliers of water treatment systems that use elemental copper for legionella control
The use of copper ionisation in legionella control
From 1 February 2013, the Biocidal Products Directive and the national Biocidal Products Regulations 2001 (which implement the Directive in Great Britain) no longer allowed the marketing and use of elemental copper as a biocide. HSE submitted an application for ‘essential use derogation’ to the EC to allow for the continued use of copper in Legionella control systems within the UK.
The Commission Decision granting the UK essential use derogation for the use of copper in Legionella control in product types 2 (control of Legionella in water for human use, such as bathing and showering water) and 5 (control of Legionella in drinking water) was published in the Official Journal of the European Union on 15 February 2014. Companies wishing to place a biocidal product (or family of products) using copper on the UK market for product types have applied to HSE for an Essential Use Product Authorisation.
The Q&A below should help address any further questions you may have. The biocides helpdesk can give further advice about the biocidal use of elemental copper via email: biocides@hse.gov.uk and the HSE Biocides web site provides more details on the biocides directive. Further information about responsibilities to control the risk of exposure to legionella is available by email from COSHH.enquiries@hse.gov.uk.
Can I view the European Commission's decision on the 'essential use' derogation application?
The UK Competent Authority for biocidal products submitted an application for an essential use derogation for the placing on the market and use of copper for biocidal product-types PT02 and 05, in accordance with article 5 of Regulation (EC) No 1451/2007 and this was formally granted by the European Commission on 15 February 2014. The Commission formally published their decision in the Official Journal of the European Union on 15 February 2014.
What does an 'essential use' derogation mean for my system?
The favourable decision by the EC for the essential use derogation application allows for the continued use of elemental copper and the placing on the market of biocidal products containing copper whilst industry provides the data required under the Directive by a specified deadline.
- Isn't it true that there's no safe limit for silver in water?
Technically true, but misleading. Far from WHO implying that no amount of silver in water is safe, it seems that any risk from silver is very low.
ReferenceAs HTM 04-01 points out, the World Health Organisation (2011) states that there is no adequate data with which to derive a health-based guideline value for silver in drinking water.In a further WHO publication in 2014, however, they stated: -
“There is currently no WHO health-based guideline value for silver in the drinking-water guidelines (WHO, 2011). Silver was last reviewed by the WHO for the drinking-water guidelines in 1993, when it was concluded that on the basis of epidemiological and pharmacokinetic knowledge at the time a total lifetime oral intake of about 10g of silver could be considered the human no observable adverse effect level (NOAEL). As it was felt that the contribution of drinking-water to this NOAEL would normally be negligible it was not deemed necessary to establish a health-based guideline value. However, it was suggested that where silver salts are used for drinking-water treatment that concentration of 0.1mg/l could be tolerated without risk to health (a concentration that would give total dose over a 70 year period of half of the NOAEL outlined above). The 0.1 mg/l level is thus a health advisory rather than a guideline value.”
(Centre for Research into Environment and Health, 2014, Silver: water disinfection and toxicity, paragraph 6.3., available at http://www.who.int/water_sanitation_health/dwq/chemicals/Silver_water_disinfection_toxicity_2014V2.pdf?ua=1)
In addition: -
“Health and Welfare Canada and the US Public Health Service recommended a maximum acceptable concentration of 50 µg/L of silver in drinking water (HWC 1978). At this concentration it would take 27 years to ingest the human toxic dose of 1 gram of silver, assuming no elimination and a 2 litre per day intake. This recommendation has since been dropped. In practice, elimination is over 90% of the ingested dose so it would take several life-times.”
(Warrington, P.D., 1996, Ambient water quality criteria for silver, para. 4.7.1, Ministry of Environment, Lands and Parks, Province of British Columbia, Canada)
- Doesn't a copper silver ionisation system stain basins?
This has been known to happen in hard water areas on occasion, but the stains are easily removed. We can recommend very effective products to do this.
- Is the Orca system as good as temperature control?
We've installed a number of systems where temperature control has failed to prevent Legionella from thriving, and controlled it successfully.
- Can the system be used with dialysis patients?
Dialysis water must be absolutely pure so any chemicals such as chlorine, fluoride, calcium, magnesium, etc. must be removed beforehand. So yes, the system can be used with dialysis patients as long as the water is properly treated. More information can be found below.
- Isn't chlorine dioxide a more effective method of water treatment?
In truth, we have installed Orca successfully in hospitals where chlorine dioxide has been tried and failed. This table provides a comparison of the systems used to control legionella.
- Does copper/silver ionisation work against other bacteria?
Yes. It's been documented as being effective against Pseudomonas aeruginosa, Stenotrophomonas maltophilia, Acinetoacter baumannii, Escherichia coli, Staphylococcus aureus and mycobacterial species.
Reference“The results showed all copper ion concentrations tested (0.1–0.8 mg/L) achieved more than 99.999% reduction of P. aeruginosa which appears to be more susceptible to copper ions than S. maltophilia and A. baumannii. Silver ions concentration of 0.08mg/L achieved more than 99.999% reduction of P. aeruginosa, S. maltophilia and A. baumannii in 6, 12 and 96 h, respectively. Combination of copper and silver ions exhibited a synergistic effect against P. aeruginosa and A. baumannii while the combination exhibited an antagonistic effect against S. maltophilia.”
Huang, H.-I. et al, (2008), In vitro efficacy of copper and silver ions in eradicating Pseudomonas aeruginosa, Stenotrophomonas maltophilia and Acinetobacter baumannii: Implications for on-site disinfection for hospital infection control, Water Research 42, p.72-80
“The antibacterial effect and mechanism of action of a silver ion solution that was electrically generated were investigated for Staphylococcus aureus and Escherichia coli by analyzing the growth, morphology, and ultra- structure of the bacterial cells following treatment with the silver ion solution. Bacteria were exposed to the silver ion solution for various lengths of time, and the antibacterial effect of the solution was tested using the conventional plate count method and flow cytometric (FC) analysis. Reductions of more than 5 log10 CFU/ml of both S. aureus and E. coli bacteria were confirmed after 90 min of treatment with the silver ion solution.”
Woo, K.J. et al, (2008), Antibacterial Activity and Mechanism of Action of the Silver Ion in Staphylococcus aureus and Escherichia coli, Applied and Environmental Microbiology Vol.74 No. 7 p.2171 - 2178
“In summary, copper/silver ion concentrations of 0.1/0.01±0.8/0.08mg/l achieved 99.9% kill of M. avium. However, M. avium was more resistant to the bactericidal effects of copper/silver ions than Legionella, requiring 100 times greater exposure time to achieve comparable kill in vitro.”
Lin, Y.-S. et al, (1998), Inactivation of Mycobacterium avium by copper and silver ions, Wat. Res. Vol. 32, No. 7, pp. 1997-2000.
- How does getting rid of biofilm help control Legionella?
It doesn't just help, it's essential. Biofilm protects not only Legionella but also Pseudomonas and other bacteria. Control measures such as high temperatures that don't remove it can only work short-term - the bacteria will quickly recolonise.
ReferenceA biofilm forms when free-floating (planktonic) microbes attach themselves to a surface. They then secrete fibres that form a sticky matrix and help them remain there. A biofilm can be as thin as a few cell layers or inches thick, depending on conditions. They’re known to harbour a range of microbes, including Legionella and Pseudomonas.
Unfortunately, bacteria in a biofilm are much harder to kill than free-floating ones, and biofilm is hard to remove. Heating water to 85°C, for example, quickly kills planktonic pseudomonas but has much less effect on those in biofilm.
Fortunately, copper/silver ionisation is highly effective against biofilm pathogens, with one study finding that Pseudomonas was completely inactivated.
Kisko, G., and Szabo-Szabo, O., 2011, Biofilm removal of Pseudomonas strains using hot water sanitation, Acta Univ. Sapientiae Alimentaria, 4, p.69-79.
Shih, L.H. and Lin, Y.E., 2010, Efficacy of Copper-Silver Ionization in Controlling Biofilm- and Plankton-Associated Waterborne Pathogens. Appl Environ Microbiol. 2010; 76(6):p. 2032-5.
- Can drinking Orca-treated water turn skin blue?
There's a condition called argyria where excessive intake of silver turns skin blue. It's impossible for Orca-treated water to cause it.
The Orca and Dialysis Units
We're often asked if the Orca system is safe to use in hospitals with dialysis units, as silver-stabilised hydrogen peroxide is known to cause major problems in this area.
The answer is a definite yes - there's a huge difference between copper and silver ionisation and silver hydrogen peroxide. The latter relies on the use of very high levels of silver salts - 8 to 12 mg/litre - to have an effect.
The levels of silver ions released onto the water by the Orca system are very low, 0.02 - 0.08 mg/litre, less than a hundredth of that used in silver hydrogen peroxide, and are readily dealt with by the dialysis filtration system. ProEconomy carried out tests in a client hospital to check this. The results are listed below:
| Sample ID | Copper (mg/L) | Silver (mg/L) |
|---|---|---|
| Renal dialysis before osmosis | 0.246 | 0.0593 |
| Max. recommended concentration (UK Renal Association) | 0.1 | 0.005 |
| Renal dialysis after osmosis | <0.003 | 0.0004 |
As can be seen, copper and silver levels are well below guideline concentrations.
At least five hospitals have had renal unit water supplies treated by the Orca copper silver ionisation system for several years without any problems arising.
Legionella Control At Low Temperatures
ProEconomy is deeply committed to sustainability and has funded research in the field. In 2016 postgraduate students from Kings College and University College London investigated the effects of an Orca system on one of Great Ormond Street Hospital's buildings. Installing the system had enabled the estates team to reduce the hot water temperature from 65°C to 45°C whilst still controlling Legionella and Pseudomonas. The students calculated that this measure reduced both energy consumption and carbon footprint by an astonishing 33%.
| Temp Reduction (kWh) | No temp Reduction (kWh) | |
|---|---|---|
| Annual Energy use (kWh) | 160,543.47 | 238,005.09 |
| Total Carbon Footprint CO2e | 46,238.22 | 68,547.99 |
| Money Spent | £17,659.78 | £26,180.56 |
| Hospital's Energy Share | 2.54% | 3.76% |
In addition to reducing the energy bill by over £8,500 per year, the hospital was able to make considerable savings by not having to purchase and maintain expensive thermostatic mixing valves.
In their sustainable development strategy, the NHS state that their aim is to reduce their carbon emissions by 28% by 2020. The table above shows that ProEconomy can play a significant role in helping to achieve that goal by controlling Legionella at lower temperatures.
Comparison of Water Treatment Methods
| Parameters | Temperature regime | Continuous dose chlorine dioxide | Copper and Silver ionisation | Hyperchlorination | Silver stabilised hydrogen peroxide | Point of Use Filters | Ozone | UV |
|---|---|---|---|---|---|---|---|---|
| Concentration recommended at outlets | <20°C cold taps >50°C hot taps >55°C healthcare hot taps Reference Health and Safety Executive, (2014) HSG274 Part 2: The control of Legionella bacteria in hot and cold water systems, para 2.6 p.8“2.6 Temperature control is the traditional strategy for reducing the risk of Legionella in water systems. Cold water systems should be maintained, where possible, at a temperature below 20 °C. Hot water should be stored at least at 60 °C and distributed so that it reaches a temperature of 50 °C (55 °C in healthcare premises) within one minute at the outlets.” |
0.5mg/l max for total levels of ClO2, chlorite and chlorate Reference
Drinking Water Inspectorate, (April 2016), List of approved products for use in public water supply in the United Kingdom, para. A.7.3, p.16 A.7.3 On-site generation of chlorine dioxide Products in this section are not fully covered by EN 12671 (Chlorine dioxide) or introduce chlorine dioxide directly into supply once generated. The following products must conform to the following conditions of use: (i) the method of use and the purity of the output of these products shall be such that, in the case of water for public supply, the water so treated meets the requirements of the relevant Regulations, and (ii) the combined concentration of chlorine dioxide, chlorite and chlorate shall not exceed 0.5mg/l as chlorine dioxide in the water entering supply. |
Copper < 0.2mg/l Silver < 0.02mg/l Reference Lin, Y.E., Stout, J.E., & Yu, V.L., (2011), Controlling Legionella in Hospital Drinking Water: An Evidence-Based Review of Disinfection Methods, Infect Control Hosp Epidemiol 32(2):166-173 “We recommend copper ion concentrations of 0.20–0.80 mg/L and silver ion concentrations of 0.01–0.08 mg/L for Legionella eradication.” |
0.5 to 1.0mg/l Reference
Health and Safety Executive, (2014) HSG274 Part 2: The control of Legionella bacteria in hot and cold water systems, para 2.111 p.38 “2.111 WHO has set a health-based guideline maximum value of 5.0 mg/l for total chlorine as a residual disinfectant in drinking water. However, it is rarely used continuously in domestic water in buildings at levels higher than 1.0 mg/l as this would render the water unpalatable and may lead to an unacceptable level of corrosion.” |
H2O2 = 30mg/l Ag = 30µg/l Reference Environmental Protection Agency, (2001) Final Report: Evaluation of the Efficacy of a New Secondary Disinfectant Formulation Using Hydrogen Peroxide and Silver and the Formulation of Disinfection By-Products Resulting From Interactions with Conventional Disinfectants |
0.2µm pore size Reference
Sheffer, P.J., Stout, J.E., Wagener, M.M. and Muder, R.R., (2005), Efficacy of new point-of-use water filter for preventing exposure to Legionella and waterborne bacteria. Am J Infect Control 33(5 Suppl 1):S20-5. “The filter provides a barrier for particles greater than 0.2 micrometers in size.” |
0.1 to 1.0 mg/l Reference
Kim BR, Anderson J E, Mueller S A, Gaines W A, Kendall A M., (2002), Literature review-efficacy of various disinfectants against Legionella in water systems, Water Research, Volume 36, Pages 4433—4444. See table on p.4440 |
254 nm Reference
Lin, Y.S., Stout, J.E., Yu, V.L. and Vidic, R.D., (1998), Disinfection of water distribution systems for Legionella, Semin Respir Infect.;13(2):147-59. “Ultraviolet light (254 nm) kills bacteria by producing thymine dimers in DNA which subsequently hampers DNA replication.” |
| Residual effect | None | Limited due to decay at hot temps Reference
Department of Health, 2016, Health Technical Memorandum 04-01: Safe water in healthcare premises, Para. 15.34, page 69. “In the case of hot water distribution systems with calorifiers/water heaters operating conventionally (that is, at 60°C), there will be a tendency for chlorine dioxide to be lost by gassing off, especially if the retention time in a vented calorifier/water heater is long.” Zhang Z, McCann C, Stout JE, Piesczynski S, Hawks R, Vidic R, Yu VL., (2007), Safety and efficacy of chlorine dioxide for Legionella control in a hospital water system. Infect Control Hosp Epidemiol. Aug;28(8):1009-12. “It is clear that maintaining a sufficient residual level of chlorine dioxide in the hot water system is challenging. An elevated water temperature hastens the conversion of chlorine dioxide to chlorite by the reactions with organic compounds in the water distribution system. This finding is consistent with our observation that the mean chlorite concentration in hot water was higher than that in cold water.” |
Prolonged Reference
Lin, Y.E., Stout, J.E., & Yu, V.L., (2011), Controlling Legionella in Hospital Drinking Water: An Evidence-Based Review of Disinfection Methods, Infect Control Hosp Epidemiol 32(2):166-173 “When the ionization unit was deliberately inactivated, recolonization was delayed, and the water system remained free of Legionella for an additional 2–3 months.” “Ionization has a prolonged efficacy that provides an added margin of safety, unlike hyperchlorination, with which Legionella can rapidly appear in the event of system malfunction. Unlike chlorine and chlorine dioxide, the biocidal activity of copper-silver ionization is not compromised by higher water temperature.” |
Yes Reference
Lin, Y.E, Vidic, R.D., Stout, J.E. and Yu, V.L., 1998, Legionella in water distribution systems., JAWWA, 90, 112-121. “Residual disinfectant is provided throughout the entire water distribution system.” |
Significant Reference
Pedazhur, R. et al, (2000), The efficacy of long-lasting residual drinking water disinfectants based on hydrogen peroxide and silver, Water Science & Technology, Vol. 42, Issue 1-2, p. 293 – 298 *Original paper not consulted, but it is referred to in this respect in Scottish Health Technical Memorandum 04-01 (2011). |
Not applicable | None Reference
Kim BR, Anderson J E, Mueller S A, Gaines W A, Kendall A M., (2002), Literature review-efficacy of various disinfectants against Legionella in water systems, Water Research, Volume 36, Pages 4433—4444. See table on p.4440 |
None Reference
Lin, Y.E, Vidic, R.D., Stout, J.E. and Yu, V.L., 1998, Legionella in water distribution systems., JAWWA, 90, 112-121. “UV light does not provide residual protection because Legionella will persist in biofilms where UV light cannot penetrate.” |
| Time to re-colonisation after system shut-down | Not known Reference
Unable to find any information on this subject from independent sources, i.e. scientific literature. |
4 days Reference
Lin, Y.E., Stout, J.E., & Yu, V.L., (2011), Controlling Legionella in Hospital Drinking Water: An Evidence-Based Review of Disinfection Methods, Infect Control Hosp Epidemiol 32(2):166-173 “It is noteworthy that on 2 occasions when the chlorine dioxide concentration fell below 0.25 mg/ L because of mechanical failure, Legionella was detected in water samples within 4 days.” |
6 - 12 weeks Reference
Liu Z., Stout, J.E., Boldin, M., Rugh, J., Diven, W.F., and Yu, V.L., (1998), Intermittent use of copper-silver ionization for Legionella control in water distribution systems: a potential option in buildings housing individuals at low risk of infection. Clin Infect Dis 26: 138–140 “Twelve weeks of disinfection reduced the distal site positivity for Legionella in the first test building to zero. Legionella recolonization did not occur in the first test building for 6-12 weeks and in the second building for 8-12 weeks after inactivation of the system.” |
Days Reference
Lin, Y.E, Vidic, R.D., Stout, J.E. and Yu, V.L., 1998, Legionella in water distribution systems., JAWWA, 90, 112-121. “If a chlorinator fails or malfunctions, Legionella can reemerge within days.” |
Significant Reference
Pedazhur, R. et al, (2000), The efficacy of long-lasting residual drinking water disinfectants based on hydrogen peroxide and silver, Water Science & Technology, Vol. 42, Issue 1-2, p. 293 – 298 *Original paper not consulted, but it is referred to in this respect in Scottish Health Technical Memorandum 04-01 (2011). |
Not applicable | Not applicable | Only works at point of entry |
| Effect on biofilm | None | Controls, Reference but may penetrate to only 100µmZhang Z, McCann C, Hanrahan J, Jencson A, Joyce D, Fyffe S, Piesczynski S, Hawks R, Stout JE, Yu VL. Legionella control by chlorine dioxide in hospital water systems. JAWWA, 2009, 101(5): 117-127 “ClO2 is effective against viruses, bacteria, protozoan cysts, biofilm, and waterborne pathogens in public drinking water systems.” Reference
Jang, A., et al, (2006), Measurement of chlorine dioxide penetration in dairy process pipe biofilms during disinfection, Appl Microbiol Biotechnol. Sep;72(2):368-76. “ClO2 profiles showed depletion of disinfectant at 100 μm in the biofilm depth, indicating that ClO2 may not reach bacteria in a biofilm thicker than this using a 25 mg/l solution.” |
Controls Reference
Shih, H.-Y. and Lin, Y.E., (2010), Efficacy of Copper-Silver Ionization in Controlling Biofilm- and Plankton-Associated Waterborne Pathogens, Applied and Environmental Biology, Mar; 76(6): 2032–2035. “In summary, copper-silver ionization is efficacious for control of biofilms and plankton-associated waterborne pathogens in a model plumbing system. Copper-silver ionization may be capable of controlling waterborne pathogens, in addition to Legionella, in the hospital water distribution system.” |
Limited Reference
De Beer, D., Srinivasan, R. and Stewart, P., (1994), Direct measurement of chlorine penetration into biofilms during disinfection, Applied and Environmental Microbiology, Vol. 60 No.12, p.4339- 4344 “Comparison of the cross sections and chlorine microprofiles leads to the conclusion that decreased action of chlorine against biofilms is due to limited penetration stemming from a reaction-diffusion interaction. The microprofiles show that, after exposure to 2.5 ppm of chlorine for 1 h, only the upper 100 μm of the cell clusters is penetrated by chlorine.” Lin, Y.E, Vidic, R.D., Stout, J.E. and Yu, V.L., 1998, Legionella in water distribution systems., JAWWA, 90, 112-121. “Because chlorine has a limited ability to penetrate biofilms, it is less effective against biofilm-associated microorganisms such as Legionella.” |
Limited effect Reference
Armon, R. et al, (2000), Controlling biofilm formation by hydrogen peroxide and silver combined disinfectant, Water Science and Technology, Vol. 42 No. 1-2, p.187-192 “Biofilm prevention effectivity of chlorine (approximately 1 ppm) was considerably higher than that of the combined disinfectant.” |
No effect | Varies Reference
Bialoszewski, D. et al, (2011), Activity of ozonated water and ozone against Staphylococcus aureus and Pseudomonas aeruginosa biofilms, Med Sci Monit. 17(11): BR339–BR344. “Freshly ozonated water can be an effective solution for destroying bacterial biofilms. In the case of biofilms formed by strains of Staphylococcus aureus, ozonated water reduced viable cell counts to background levels following very brief exposures (30 seconds). Some cell populations of the strains of P. aeruginosa investigated in the present study exhibited tolerance to the action of ozonated water.” Hems, R.S., Gulabivala, K., Ng, Y-.L., Ready, D. and Spratt, D.A., (2005), An in vitro evaluation of the ability of ozone to kill a strain of Enterococcus faecalis, International Endodontic Journal, 38, 22–29 “Biofilms incubated for 240s with ozonated water showed no significant reduction in cell viability attributable to ozone alone, whereas with NaOCl no viable cells were detected over the same time. Gaseous ozone applied for 300 s had no effect on these biofilms. Ozone had an antibacterial effect on planktonic E. faecalis cells and those suspended in fluid, but little effect when embedded in biofilms.” |
Only works at point of entry Reference
Lin, Y.E, Vidic, R.D., Stout, J.E. and Yu, V.L., 1998, Legionella in water distribution systems., JAWWA, 90, 112-121. “UV light does not provide residual protection because Legionella will persist in biofilms where UV light cannot penetrate.” |
| Efficacy affected by temperature | Not applicable | Decays at hot temperatures Reference
Zhang, Z., McCann, C., Hanrahan, J., Jencson, A., Joyce, D., Fyffe, S., Piesczynski, S., Hawks, R., Stout, J.E. and Yu, V.L., (2009), Legionella control by chlorine dioxide in hospital water systems. JAWWA, 101(5): 117-127. “However, an extended period (18 months) was needed to achieve this reduction in positivity, which is most likely due to the low concentration of ClO2 in the hot water. This is significant because Legionella species proliferate in hot water (Lin et al, 1998b). It is clear that maintaining sufficient ClO2 residual in the hot water system is challenging. Elevated water temperature hastens the conversion of ClO2 to ClO2– through the reactions with organic compounds in the water distribution system.” |
Unaffected Reference
Lin, Y.E., Stout, J.E., & Yu, V.L., (2011), Controlling Legionella in Hospital Drinking Water: An Evidence-Based Review of Disinfection Methods, Infect Control Hosp Epidemiol 32(2):166-173 “Unlike chlorine and chlorine dioxide, the biocidal activity of copper-silver ionization is not compromised by higher water temperature.” |
Compromised Reference
Lin, Y.E., Stout, J.E. and Yu, V.L., (1998), Disinfection of water distribution systems for Legionella. Semin Resp Infect,13:147-159. “In addition, chlorine decomposes at increased water temperatures.” |
Activity doubles by increasing temp. from 4° to 24° Reference
Environmental Protection Agency, (2001), Final Report: Evaluation of the Efficacy of a New Secondary Disinfectant Formulation Using Hydrogen Peroxide and Silver and the Formulation of Disinfection By-Products Resulting From Interactions with Conventional Disinfectants “Inactivation performance increased with temperature (e.g., two-fold increase in log inactivation of E. coli resulted by increasing the temperature from 4 to 24 C for a 1-hour exposure to the optimized formulation).” |
Unaffected | Unaffected Reference
Muraca, P., Stout, J.E. and Yu, V.L., (1987), Comparative assessment of chlorine, heat, ozone and UV light for killing Legionella pneumophila within a model plumbing system, Appl. Environ. Microbiol, vol.53, no. 2, p. 447-453 “Neither turbidity nor the higher temperature of 43 degrees C impaired the efficacy of any of the disinfectant methods.” |
Unaffected Reference
Muraca, P., Stout, J.E. and Yu, V.L., (1987), Comparative assessment of chlorine, heat, ozone and UV light for killing Legionella pneumophila within a model plumbing system, Appl. Environ. Microbiol, vol.53, no. 2, p. 447-453 “Neither turbidity nor the higher temperature of 43 degrees C impaired the efficacy of any of the disinfectant methods.” |
| Efficacy affected by pH | No | Effective pH5 to pH10 Reference
LeChevallier, M.W. and Au, K-K, (2004), Water Treatment and Pathogen Control, WHO, p.52 “Chlorine dioxide is highly soluble in water (particularly at low temperatures), and is effective over a range of pH values (pH 5–10).” |
Elevated pH (>8.5) affects efficacy in hard water areas Reference
Lin, Y.S., Stout, J.E., and Yu, V.L., (2002), Negative effect of high pH on biocidal efficacy of copper and silver ions in controlling Legionella pneumophila. Appl Environ Microbiol 68:2711– 2715. “When the pH was elevated to 9 in these experiments, copper ions achieved only a 10-fold reduction in the number of Legionella organisms in 24 h, compared to a millionfold decrease at pH 7.0.” |
Not known Reference
Unable to find any information on this subject from independent sources, i.e. scientific literature. |
Activity increases pH6 to pH9 Reference
Environmental Protection Agency, (2001), Final Report: Evaluation of the Efficacy of a New Secondary Disinfectant Formulation Using Hydrogen Peroxide and Silver and the Formulation of Disinfection By-Products Resulting From Interactions with Conventional Disinfectants “Inactivation performance of the combined disinfectant increased at basic pH (e.g., log activation increased by two-fold by increasing the pH from 6 to 9 using the optimized formulation).” |
Unaffected | Unaffected Reference
Domingue, E.L. et al, (1988), Effects of three oxidizing biocides on Legionella pneumophila serogroup 1, Appl Environ Microbiol. Mar;54(3):741-7. “The bactericidal action of O3 was not markedly affected by changes in pH or temperature.” |
No |
| Adverse affects | Scalding risk Reference Blended water is not protected Department of Health, 2016, Health Technical Memorandum 04-01: Safe water in healthcare premises Part A, para. 10.59, p. 54 “The risk of scalding for vulnerable patients (young children and older people, disabled people and those with neuropathy) is of particular concern in healthcare premises caring for such individuals, and therefore thermostatic mixing devices could be needed for hot water outlets. A risk assessment for scalding risk versus the risk of infection from waterborne pathogens should be undertaken by the WSG.” Reference Hot temperatures can be a contributory factor to corrosion Temperatures that are hot enough to kill bacteria will also harm humans and so must be reduced where required. Unfortunately, this brings them within the range where pathogens can thrive. What’s more, the thermal mixing valves used to blend hot and cold water can act as a source of Legionella and other pathogens. Reference
Oliphant, R.J., (rev. 2010), Causes of copper corrosion in plumbing systems (FR/R0007), Foundation for Water Research. See Table 1. Types and causes of pitting corrosion |
Hazardous Chlorate and chlorite Reference Adverse effect on neonates World Health Organisation, (2005), Chlorate and chlorite in drinking water “Chlorine dioxide rapidly decomposes into chlorite, chlorate and chloride ions in treated water, chlorite being the predominant species.” Reference Corrosive Tuthill, R.W., Giusti, R.A., Moore, G.S. and Calabrese, E. J., (1982) Health affects among newborns after parental exposure to ClO2 disinfected drinking water. Environmental Health Perspectives. Volume 46, Pages 38-39.“A statistically significant positive association was found between exposure of the mother to ClO2-treated water during pregnancy and prematurity of the newborn as assessed by the attending physician and by a greater weight loss after birth.”Kanitz, S., Franco, Y., Patrone, V., Caltabellotta, M., Raffo, E., Riggi, C., Timitilli, D. and Ravera, G., (1996), Association between drinking water disinfection and somatic parameters at birth, Environ Health Perspect. 104(5): 516–520.“Results indicate a higher frequency of small body length (< or = 49.5 cm) and small cranial circumference (< or = 35 cm) in infants born to mothers who drank water treated with chlorine compounds. In particular, the statistical analysis (by simultaneous variance analysis and Scheffé test) indicated that there may be an association between infants with smaller body length and mothers who drank water treated with chlorine dioxide [adjusted odds radio (OR) = 2.0; 95% CI = 1.2-3.3] or sodium hypochlorite (adjusted OR = 2.3; 95% CI = 1.3-4.2) and between infants with smaller cranial circumference and mothers who drank water treated with chlorine dioxide (adjusted OR = 2.2; 95% CI = 1.4-3.9) or sodium hypochlorite (adjusted OR = 3.5; 95% CI = 2.1-8.5). The presence of neonatal jaundice is almost twice as likely (adjusted OR = 1.7; 95% CI = 1.1-3.1) in infants whose mothers drank water treated with chlorine dioxide.” Reference
a) Department of Health, 2016, Health Technical Memorandum 04-01: Safe water in healthcare premises, Para. 15.35, Note 2, page 69.“Excessive values of chlorine dioxide should be avoided subsequently since it can corrode copper and steel pipework and can also damage non-metallic pipework and component parts, particularly at higher temperatures.”b) Duvall, D.E. and Edwards, D.B., (2010), Forensic Analysis of Oxidation Embrittlement in Failed HDPE Potable Water Pipes, Proceedings of Pipeline Division Specialty Conference, American Society of Civil Engineers (ISBN: 978-1-61738-985-6)“1) The finite supply of anti-oxidants (AO) included in the HDPE pipe formulation are consumed on the inner pipe surface both by being washed off that surface by flowing water and by chemical reaction with a continuous supply of oxidant in the form of water disinfectants continually flowing through the pipe. Additional AO in the bulk of the pipe wall is consumed as it migrates from the pipe core to the areas of reaction on the inner surfaces.
2) When the protective AO package is exhausted or depleted, the water disinfectant oxidants degrade the polymer at the pipe inner surface. This degradation is characterized by a reduced molecular weight and diminished mechanical properties of the polymer at that surface. 3) When degradation of the inner surface material is severe enough, the embrittled surface layer develops cracks which will propagate through the pipe wall, driven by internal pressure and other sources of pipe wall stress. The result of this process will be Stage III non-ductile failure of the HDPE pipe. The oxidative embrittlement of HDPE pipe through exposure to water disinfectants is significant in that crack initiation in non-degraded pipe (non-chemical processes) may account for up to 90% of the total lifetime of a pipe. Therefore, overall HDPE service life could be dramatically reduced by inner pipe wall surface oxidation.” “Chlorine dioxide has been shown to be the most aggressive oxidizing agent with respect to polyethylene pipe while chloramines have appeared to be the least aggressive.” c) Health and Safety Executive, (2014) HSG274 Part 2: The control of Legionella bacteria in hot and cold water systems, para. 2.98, p.36 “2.98 Excessive levels of chlorine dioxide should be avoided since they can encourage the corrosion of copper and steel pipework and high levels of chlorine dioxide can degrade certain types of polyethylene pipework particularly at elevated temperatures. Users of chlorine dioxide systems will need to consider these issues and when choosing a system these points should be checked to ensure that the supplier addresses them satisfactorily.” |
Staining of sanitary ware in hard water areas Reference
Department of Health, 2016, Health Technical Memorandum 04-01: Safe water in healthcare premises Part A, para. 4.19, p. 25 “There have been cases of staining to sanitaryware in hard water areas.” |
Hazardous Produces trihalomethanes (THMs), possible carcinogens Reference Highly corrosiveOrsi, G. et al, (2014) Legionella control in the water system of antiquated hospital buildings by shock and continuous hyperchlorination: 5 years experience, BMC Infectious Diseases, 14, 394 “In our experience, continuous free chlorine levels between 0.5 and 1.0 mg/L (Figure 3) were effective in reducing significantly Legionella presence in the old hospital water system. Unfortunately the continuous hyperchlorination at >0.5 < 1.0 mg/L determined the non-potability of drinking water [16], and the production of disinfectant by-products such as trihalomethanes increased.” Reference Lin, Y.E., Stout, J.E., & Yu, V.L., (2011) Controlling Legionella in Hospital Drinking Water: An Evidence-Based Review of Disinfection Methods, Infect Control Hosp Epidemiol 32(2):166-173“Hyperchlorination was found to be the most unreliable and also the most expensive disinfection modality. It has met with increasing disfavor because of inadequate penetration of the agent into biofilms in piping, persistence of Legionella organisms in hyperchlorinated systems,59 corrosion of the water distribution system leading to pinhole leaks over time, and the introduction of carcinogens into the drinking water.60”Lin, Y.E, Vidic, R.D., Stout, J.E. and Yu, V.L., 1998, Legionella in water distribution systems., JAWWA, 90, 112-121.“Chlorine is highly corrosive and damages pipes. Three years after chlorination at the University of Iowa hospital, the incidence of pipe leaks was 30 times the rate before chlorination.” |
Hydrogen peroxide is known to be dangerous to renal dialysis patients Reference
National Patient Safety Agency, 2008 Risk to haemodialysis patients from water supply (hydrogen peroxide), Rapid Response Report NPSA/2008/RRR007 |
The structure and composition of POU filters can promote biofilm formation | Hazardous Can increase corrosion Reference U.S. Environmental Protection Agency (USEPA), (2007), Simultaneous compliance Guidance Manual for the long term 2 and stage 2 DBP rules, USEPA, Washington DC, USA, p.5-16 “Can increase corrosion.” |
None known |
| Other issues | No efficacy field studies have been carried out Pathogens survive <20°C and >55°C |
Pathogens survive 4 to 5mg/l Reference Long time needed to obtain control Mustapha, P., Epallet, T., Allegra, S., Girardot, f., Garraud, O. and Riffard, S., (2015), Monitoring of Legionella pneumophila viability after chlorine dioxide treatment using flow cytometry, Res Microbiol, 166 (3), 215-219. “The viability of three Legionella pneumophila strains was monitored after chlorine dioxide (ClO2) treatment using a flow cytometric assay. Suspensions of L. pneumophila cells were submitted to increasing concentrations of ClO2. Culturable cells were still detected when using 4 mg/L, but could no longer be detected after exposure to 6 mg/L of ClO2, although viable but not culturable (VBNC) cells were found after exposure to 4-5 mg/L of ClO2. When testing whether these VBNC were infective, two of the strains were resuscitated after co-culture with Acanthamoeba polyphaga, but neither of them could infect macrophage-like cells.” Reference
Sidari, F.P., Stout, J.E., Vanbriesen, J.M. et al, (2004), Keeping Legionella out of water systems. J Am Water Works Assoc 96:111–119 “Eradication of Legionella was achieved after 1.75 years.” Srinivasan, A., Bova, G., Ross, T. et al, (2003), A 17-month evaluation of a chlorine dioxide water treatment system to control Legionella species in a hospital water supply. Infect Control Hosp Epidemiol 24:575–579. “Seventeen months of continuous operation of such a system in one building at our institution has nearly eradicated Legionella from the building’s water supply.” Casini, B., Valentini, P., Baggiani, A. et al, (2008), Molecular epidemiology of Legionella pneumophila serogroup 1 isolates following long-term chlorine dioxide treatment in a university hospital water system, J Hosp Infect 69:141–147 “The impact of the safety plan on the ecology of Legionella in the water network was evaluated by studying the genetic variability and the chlorine susceptibility of the strains isolated prior to, and throughout, the application of continuous chlorine dioxide treatment. After 45 months of water hyperchlorination, Legionella spp. were still present.” |
Electrodes scale up in hard water areas at > pH7.6 - needs regular cleaning Reference
Health and Safety Executive, (2014) HSG274 Part 2: The control of Legionella bacteria in hot and cold water systems, paras. 2.107 and 2.108, p.38 “2.107 The copper and silver ionisation system should be regularly inspected and its electrodes cleaned as required to ensure that the system is delivering steady levels of more than 0.2 mg/l copper and more than 0.02 mg/l silver, measured at outlets, necessary to maintain control. Water samples should be taken regularly from the ionisation system and from the sentinel outlets and analysed by a UKAS-accredited laboratory to ensure enough copper and silver is produced by the system. 2.108 For most systems, routine inspection and maintenance is usually sufficient to ensure control and any remedial action should be taken when necessary and recorded:
Additional mineral ions present in hard water are attracted to the electrodes, so causing a greater level of scaling. The unique features of the Orca system, however, reduce the level of this scaling considerably. |
No efficacy field studies have been carried out | No efficacy field studies have been carried out | Filters need regular replacement Reference
Health and Safety Executive, (2014) HSG274 Part 2: The control of Legionella bacteria in hot and cold water systems, table 2.1 p.32 “Record the service start date and lifespan or end date and replace filters as recommended by the manufacturer (0.2μm membrane POU filters should be used primarily as a temporary control measure while a permanent safe engineering solution is developed, although long-term use of such filters may be needed in some healthcare situations).” |
No efficacy field studies have been carried out | Fouling - lamps need regular maintenance Reference
Liu et al, (1995), Efficacy of ultraviolet light in preventing Legionella colonization of a hospital water distribution system, Wat. Res. Vol 29, No.10, p. 2275 – 2280. “Proper maintenance and cleaning of u.v. lamps is necessary.” |
| Summary | Relatively ineffective, difficult to maintain, no effect on biofilm | Reasonably effective but quickly gasses off at higher temperatures and over distance, toxic, corrosive | Effective, safe, long residual effect. Can be affected by pH > 8.5 | Limited effectiveness on bacteria and biofilm, hazardous, highly corrosive | Reasonably effective but no effect on biofilm, H2O2 dangerous to renal patients | Only effective at point of use, no effect on biofilm, expensive, high maintenance, reduced flow | Effective at killing bacteria, but degrades very rapidly and corrodes steel pipes, rubber, etc. | Only effective at point of use, no effect on system biofilm |
Maintenance Of Our Water Treatment System
We believe that any water treatment modality needs to be correctly maintained and looked after.
This is why any copper silver ionisation system that we install comes with maintenance packages. Having a ProEconomy maintenance package includes all repairs of any broken or faulty piece of equipment (see our standard terms and conditions). It does not include upgrades.
Our maintenance package is very comprehensive and includes:
- Orca system metal sampling to ensure that the system is dosing at the correct levels.
- Tank metal samples to ensure that the metals are reaching the tanks at the correct dosages.
- Copper and silver electrode replacement.
- Copper and silver electrode cleaning.
- Leak repair.
- Broken Orca equipment repair.
- Orca system data download and analysis.
- Integration with Tetras, ProEconomy’s Cloud Based Water Management System.
Water Sampling and Management
We believe that it is very important in any water system to sample for disease causing bacteria and any biocide that you add to the water system.
This is why any system that we install comes with a water sampling package. It is in this way that we can:
- Ensure the efficacy of our product.
- Be aware of and resolve any water quality issues that may occur.
- Better understand the dynamics of a water system.
- Comply with regulations.
- Ensure Legionella, Pseudomonas and other water borne pathogens are controlled.
Our Tetras water management system also comes free with the purchase of any Orca system. Our water management system gives you full, transparent access to your individual water sample details and site-wide results so any water quality issues are detected and treated before contamination occurs. In this way, our Orca system combined with our Tetras water management system can provide you with an advanced water treatment and management service.