Following the publication of the new updated version of the HTM04-01 earlier this year, it is now necessary to ensure safety of all water used by patients, residents, staff and visitors. This minimises the risk of infection associated with all waterborne pathogens. This applies to other waterborne pathogens, not just Legionella. Therefore, safety procedures also apply to Pseudomonas aeruginosa, Stenotrophomonas maltophilia and Mycobacteria.
What Is Stenotrophomonas Maltophilia?
S. maltophilia is an emerging multi-drug-resistant global opportunistic pathogen. The increasing incidence of nosocomial and community-acquired S. maltophilia infections is of particular concern for immuno-compromised individuals, as this pathogen is associated with a significant fatality/case ratio (Brooke, 2012).
S. maltophilia (formerly Pseudomonas maltophilia and Xanthomonas maltophilia) lives mainly in soil and water but also animals and foods. It is more common in soil, specifically in plant rhizosphere. This is due to the high content of sulphurated amino acids in root exudates, which happen to be growth factors for S. Maltophilia (Bollet et a.l 1994). We associate S. maltophilia with wet surfaces and aqueous solutions. Cells of S. maltophilia have the ability to survive with minimal nutrients, e.g., in drinking water, ultrapure water, treated water (after water treatment of filtration, reverse osmosis, UV exposure, or deionization) and dialysate effluent.
Stenotrophomonas Maltophilia Sampling Methods
Not a lot of publications discuss methods for analysis of this pathogen. However, I think it is important to know how to sample and analyse, especially after the updated HTM. For this reason, I carried out a search to find a method of analysis for this bacterium. I found a method for isolation of the bacterium in soil samples (Bollet et al 1995), but I think it can be adapted for water.
The method makes use of both resistance to imipenem and its requirement for methionine. It used 34 soil samples from France, Vietnam, China, Hong Kong, Morocco and Ivory Coast.
Procedure
1 g of soil was placed in a tube containing 10 ml of nutrient broth (bioMe´rieux, Marcyl’Etoile, France) with 0.5 mg/ml of DL-methionine (Sigma Chemical Co., St. Louis, Mo).
After 24 h of incubation at 30 ºC, 0.1 ml of the broth was inoculated on a 90-mm-diameter Mueller-Hinton agar plate with a spreader.
Within 15 min after inoculation, four disks impregnated with 10 mg of imipenem (bioMe´rieux) each were applied to the surface of the inoculated plates.
After 18 h of incubation at 30 ºC, colonies that grew in the inhibition areas of the disks were re-isolated and identified by using the API 20E identification system (bioMe´rieux) according to the manufacturer’s instructions.
By using this technique, researchers were able to isolate 21 imipenem-resistant strains and all of them were S. maltophilia strains. These were compared with clinical isolates by using the Biotype 100 auxanogram system (API bioMe´rieux, La Balme-les-Grottes, France). There was no difference in vigour or pattern between the strains from hospitalized patients and the strains from soils.
To establish the sensitivity of the method, the researchers inoculated tubes containing 1 g of sterile soil with 10-fold successive dilutions of a suspension containing 4000 cfu of S.maltophilia.
To pinpoint inter strain variations, three strains isolated from different soils (strain A from France, strain B from Vietnam, and strain C from Morocco) were studied. Colonies that grew in the inhibition area were counted. The sensitivity ranged from 10 to 100 cfu/g of soil, depending on the strain.
The authors concluded that the method described in their article is simple, specific, sensitive, and inexpensive. By using successive dilutions of the soil samples, semi-quantitative results could be obtained.
How To Control S. Maltophilia
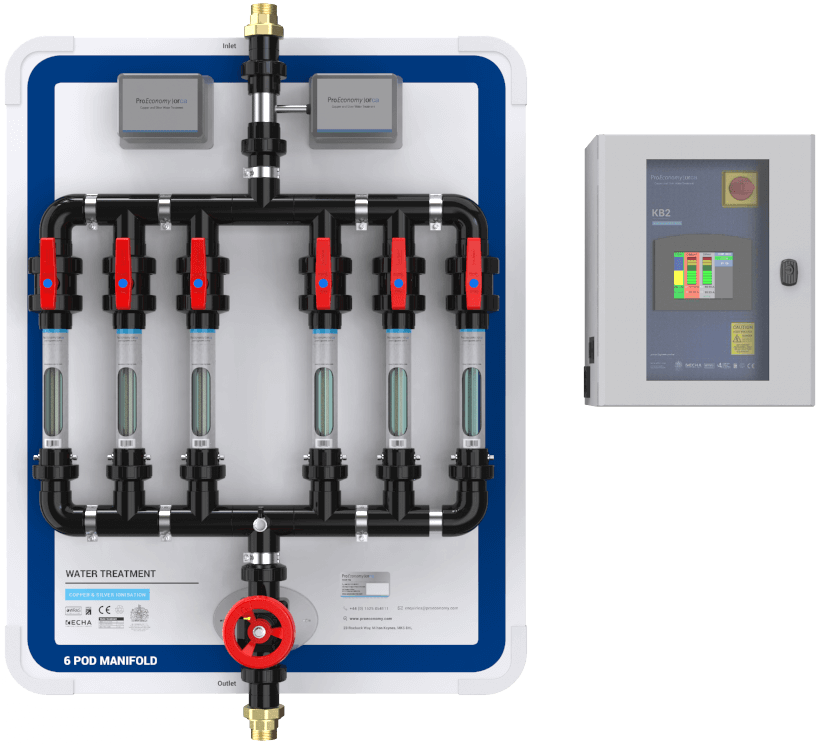
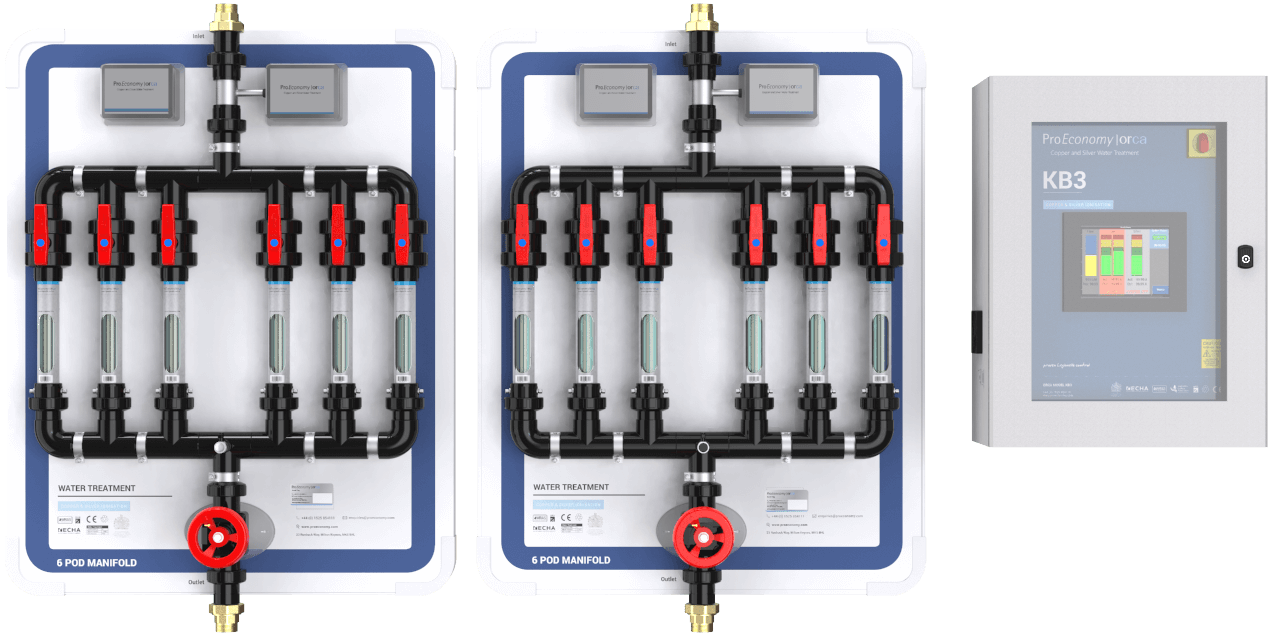
Copper and silver ionisation is effective against Legionella, P. aeruginosa, Mycobacteria, as well as S. maltophilia, and independent of water temperatures (Liu et al. 1994, Miuetzner et al. 1997, Liu et al., 1998, Stout et al. 1998, Biurrun et al. 1999, Rohr et al. 1999, Kusnetsov et al. 2001, Stout and Yu 2003, Chen et al. 2008, Lin et al, 2011, Bedford 2012).
References
Bedford B (2012) Legionella control in water systems using copper and silver ion generation systems. PhD thesis, Cranfield University, UK. Available from: https://dspace.lib.cranfield.ac.uk/bitstream/1826/7983/1/Birgitta_Bedford_Thesis_2012.pdf
Biurrun A., Caballero L., Pelaz C., Leon E., Gago A. (1999). Treatment of a Legionella pneumophila colonized water distribution system using copper-silver ionization and continuous chlorination. Infect. Control. Hosp. Epidemiol. 20:426-428.
Bollet C., Davin-Regli A., De Micco P. (1995) A simple method for selective isolation of Stenotrophomonas maltophilia from environmental samples. Applied and Environmental Microbiology 61(4):1653–1654. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC1388425/pdf/hw1653.pdf
Brooke J.S. (2012) Stenotrophomonas maltophilia: an emerging global opportunistic pathogen. Clin Microbiol Rev 25(1):2–41. doi: 10.1128/CMR.00019-11
Chen Y.S., Lin Y.E., Liu Y.C., Huang W.K., Shih H.Y., Wann S.R., Lee S.S., Tsai H.C., Li C.H., Chao H.L., Ke C.M., Lu H.H., Chang C.L. (2008). Efficacy of point-of-entry copper-silver ionisation system in eradicating Legionella pneumophila in a tropical tertiary care hospital: implications for both hospitals contaminated with Legionella in both hot and cold water. Journal of Hospital Infection. 68:152-158.
Kusnetsov J., E. Iivanainen E., Elomaa N., Zacheus O., Martikainen P.J. (2001). Copper and silver ions more effective against Legionellae then against Mycobacteria in a hospital warm water system. Wat. Res. 35(17):4217-4225.
Lin Y., Stout J.E., Yu V.L. (2011). Controlling Legionella in Hospital Drinking Water: An Evidence-Based Review of Disinfection Methods. Infection Control and Hospital Epidemiology. 32(2):166-173.
Liu Z., Stout J.E., Tedesco L., Boldin M., Hwang C., Diven W.F., Yu V.L. (1994). Controlled evaluation of copper-silver ionisation in eradicating Legionella from a hospital water distribution system. J. Infectious Disease. 169:919-922.
Liu Z., Stout J.E., Boldin M., Rugh J., Diven W.F. Yu V.L. (1998). Intermittent use of copper-silver ionisation for Legionella control in water distribution systems: A potential option in buildings housing individuals at low risk of infection. Clinical Infectious Diseases. 26:138-140.
Miuetzner S., Schwille R.C., Farley A., Wald R.R., Ge J.H., States S.J., Libert T., Wadowsky R.M. (1997). Efficacy of thermal treatment and copper-silver ionization for controlling Legionella pneumophila in high-volume hot water plumbing systems in hospitals. The Association for Professionals in Infection Control and Epidemiology, Inc. 17(46):813-866.
Stout J. E., Yu V.L. (2003). Experiences of the first 16 hospitals using copper silver ionisation for Legionella control: Implications for the valuation of other disinfection modalities. Infection Control and Hospital Epidemiology. 24(8):563-568.
Stout J.E., Lin Y.E., Goetz A.M., Muder R.R. (1998). Controlling Legionella in hospital water systems: Experience with the superheat-and-flush method and copper silver ionization. Infection control and Hospital Epidemiology. 19(12):911-914.